by
MIT scientists have discovered that light can cause evaporation at a rate beyond what is possible with heat alone, especially in water bound to the hydrogel. This “photomolecular effect” could revolutionize solar desalination and climate modelling, potentially tripling water production in desalination processes and developing solar cooling technologies.
A newly identified process could explain a variety of natural phenomena and enable new approaches to water desalination.
Evaporation happens all around us all the time, from the sweat cooling our bodies to the dew burning in the morning sun. But science’s understanding of this ubiquitous process may have been missing part of it all this time.
In recent years, some researchers have been puzzled to discover that the water in their experiments, which was contained in a sponge-like material known as a hydrogel, was evaporating at a higher rate than could be explained by the amount of heat, or thermal energy. , that the water was receiving. The surplus was large – double, or even triple, or more, the theoretical maximum rate.

When water and air interact, light can, under certain conditions, trigger evaporation without the need for heat, according to an MIT study.
Detection of light-induced evaporation
After conducting a series of new experiments and simulations, and re-examining some results from different groups that claimed to have exceeded the thermal limit, a team of researchers concluded… Massachusetts Institute of Technology He came to a startling result: under certain conditions, at the interface where water meets air, light can directly cause evaporation without the need for heat, and in fact does so more efficiently than heat. In these experiments, water was trapped in a hydrogel, but the researchers point out that this phenomenon may occur in other circumstances as well.
The results are published this week in a paper in With peopleby MIT postdoctoral researcher Yaodong Tu, mechanical engineering professor Gang Chen, and four others.

In the lab, the researchers observed the surface of the hydrogel, a JELL-O-like matrix composed mostly of water bound to a sponge-like network of thin films. These images show prepared hydrogel samples, where the top row shows the frozen (A) or dried (C, E, G) states, and the bottom row shows the “swollen states.” Credit: Courtesy of researchers
This phenomenon may play a role in the formation and evolution of fog and clouds, and thus it will be important to incorporate it into climate models to improve them. AccuracyResearchers say. It may play an important role in many industrial processes, such as solar water desalination, and may provide alternatives to the step of converting sunlight into heat first.
Implications for the research
The new results come as a surprise because water itself does not absorb light to any great extent. That’s why you can see clearly through many feet of clean water to the surface below. So, when the team first began exploring the process of solar evaporation for desalination, they first placed particles of a black, light-absorbing material in a bowl of water to help convert sunlight into heat.
The team then came across the work of another group that had achieved a double thermal limit evaporation rate, which is the highest possible amount of evaporation that can occur for a given heat input, based on basic physical principles such as conservation of heat. of energy. In these experiments, water was bonded to the hydrogel. Although they were skeptical at first, Chen and Tu began their own experiments with hydrogels, including a piece of the material from the other group.
“We tested it under a solar simulator, and it worked,” Chen says, confirming the unusually high evaporation rate. “So, we believe them now.” Chen and Tu then began making and testing their own hydrogels.
They began to suspect that the excess evaporation was caused by the light itself, and that the light photons were actually expelling beams of water molecules from the water’s surface. This effect will only occur at the boundary layer between water and air, on the surface of the hydrogel material, and possibly also on the surface of the sea or the surfaces of droplets in clouds or fog.
In the lab, they monitored the surface of the hydrogel, a JELL-O-like matrix composed mostly of water bound to a sponge-like network of thin films. They measured their responses to simulated sunlight at precisely controlled wavelengths.
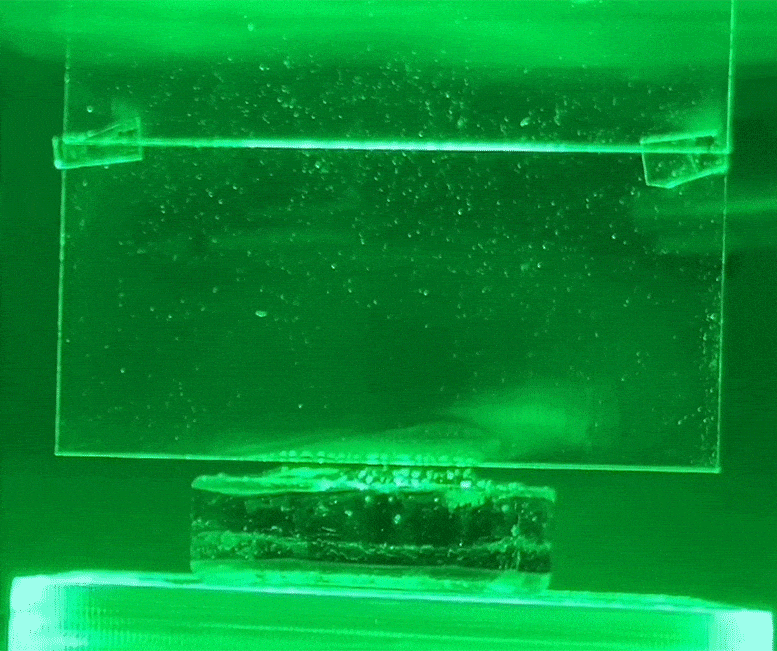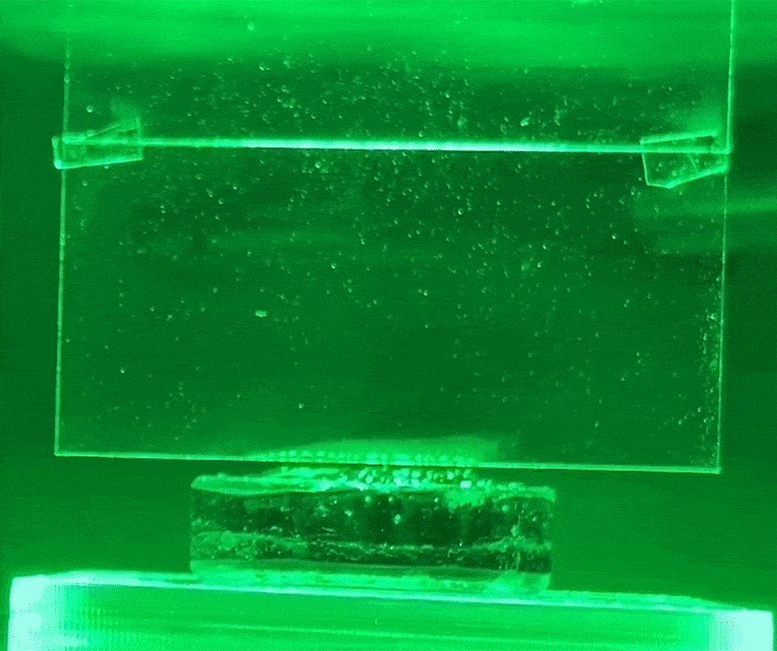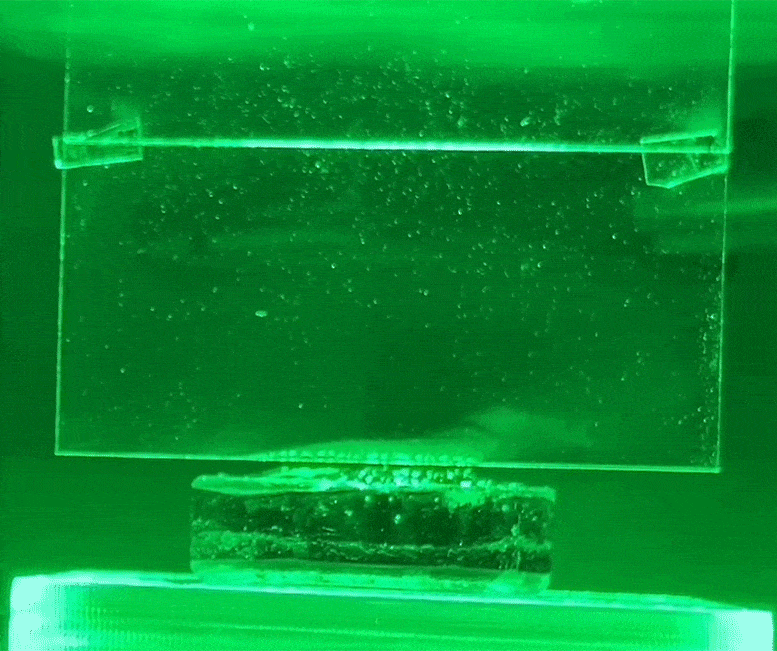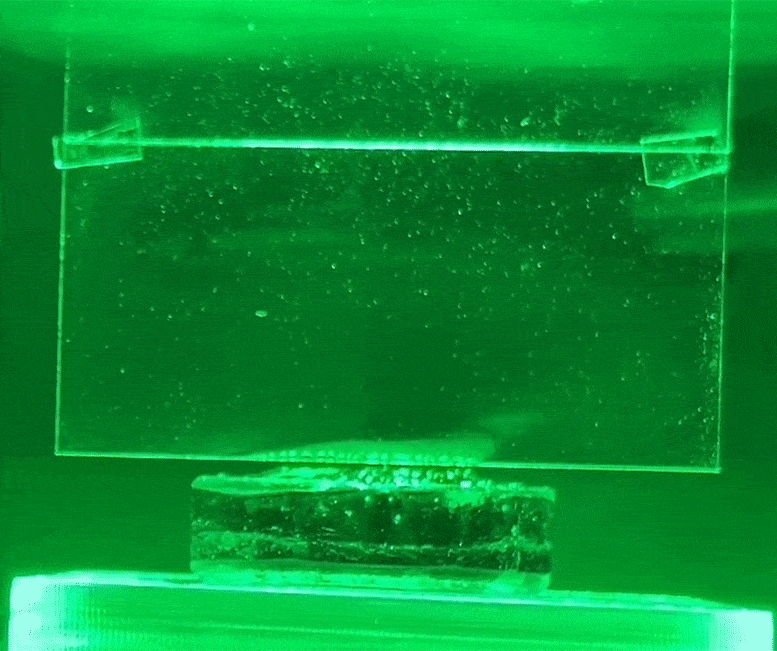
The white condensation jet on the glass is water evaporating from the hydrogel using green light, without heat. Credit: Courtesy of researchers
The researchers exposed the water surface to different colors of light sequentially and measured the evaporation rate. They did this by placing a water-filled container of hydrogel on a scale and directly measuring the amount of mass lost due to evaporation, as well as monitoring the temperature above the hydrogel’s surface. The lights were shielded to prevent them from getting overheated. The researchers found that the effect varies depending on the color and peaks at a certain wavelength of green light. This dependence on color has nothing to do with temperature, and thus supports the idea that it is light itself that causes at least some of the evaporation.
The researchers tried to replicate the observed evaporation rate with the same setup but using electricity to heat the material, without light. Although the heat input was the same as in the other test, the amount of water evaporated never exceeded the thermal limit. However, it did so while the sunlight simulation was running, confirming that light was the cause of the excess evaporation.
Although the water itself doesn’t absorb as much light as the hydrogel itself, when the two come together they become powerful absorbers, Chen says. This allows the material to efficiently harness the energy of solar photons and exceed the thermal limit, without requiring any dark pigments for absorption.
Potential applications and ongoing collaboration
Having discovered this effect, which they call the photomolecular effect, researchers are now working on how to apply it to real-world needs. They received a grant from MIT’s Abdul Latif Jameel Water and Food Systems Laboratory to study the use of the phenomenon to improve the efficiency of solar-powered desalination systems, and a Bose grant to explore the effects of the phenomenon on climate change modeling.
Tu explains that in standard desalination processes, “the desalination process usually consists of two steps: first we evaporate the water into steam, and then we need to condense the steam to liquefy it into fresh water.” With this discovery, he says, we will likely “be able to achieve high efficiency on the evaporation side.” The process can also have applications in processes that require drying of the material.
Chen says he believes in principle it may be possible to increase the maximum water produced by solar desalination, which currently stands at 1.5 kilograms per square meter, by up to three or four times using this light-based approach. “This could actually lead to cheap water desalination,” he says.
Tu adds that this phenomenon could also be harnessed in evaporative cooling processes, using phase change to provide a highly efficient solar cooling system.
At the same time, researchers are also working closely with other groups trying to replicate the results, hoping to overcome the skepticism faced by the unexpected results and the hypothesis that is advanced to explain them.
Reference: “Reasonable photomolecular effect leading to water evaporation beyond the thermal limit” by Yaodong Tu, Jiawei Zhou, Xiaoting Lin, Mohamed Al-Sharrah, Xuan Zhao, and Zhang Chen, 30 October 2023, Proceedings of the National Academy of Sciences.
doi: 10.1073/pnas.2312751120
The research team also included Jiawei Zhou, Shaoting Lin, Mohamed Al-Sharrah, and Xuanhe Zhao, all in the MIT Department of Mechanical Engineering.

“Infuriatingly humble alcohol fanatic. Unapologetic beer practitioner. Analyst.”
